
| |
| |
| |
| |
| |
| |
| |
qualitative and quantitative analysis of crystalline compounds. The
technique provides information that cannot be obtained any other way. The
information obtained includes types and nature of crystalline phases
present, structural make-up of phases, degree of crystallinity, amount of
amorphous content, microstrain & size and orientation of crystallites.
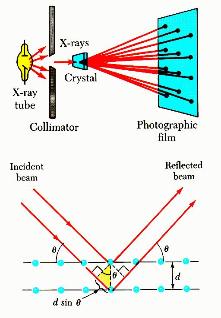
monochromatic X-rays, the atomic lattice of the sample acts as a three
dimensional diffraction grating causing the X-ray beam to be diffracted to
specific angles. The diffraction pattern, that includes position (angles) and
intensities of the diffracted beam, provides several information about the
sample and are discussed below:
- Angles are used to calculate the interplanar atomic spacings (d-
spacings). Because every crystalline material will give a characteristic
diffraction pattern and can act as a unique ‘fingerprint’, the position (d)
and intensity (I) information are used to identify the type of material by
comparing them with patterns for over 80,000 data entries in the
International Powder Diffraction File (PDF) database, complied by the
Joint Committee for Powder Diffraction Standards (JCPDS). By this
method, identification of any crystalline compounds, even in a complex
sample, can be made. - The position (d) of diffracted peaks also provides information about
how the atoms are arranged within the crystalline compound (unit cell
size or lattice parameter). The intensity information is used to assess
the type and nature of atoms. Determination of lattice parameter helps
understand extent of solid solution (complete or partial substitution of
one element for another, as in some alloys) in a sample. - Width of the diffracted peaks is used to determine crystallite size and
micro-strain in the sample. - The ‘d’ and ‘I’ from a phase can also be used to quantitatively estimate
the amount of that phase in a multi-component mixture.
No problem is too big.
We are the experts in
materials testing and failure
analysis.
(800) 682 - 2922
but also for quantitative estimation of various crystalline phases. This is one
of the important advantage of X-ray diffraction technique. Several methods
have been proposed and successfully used to quantify crystalline phases in
mixtures. They include external standard methods, the
reference-intensity-ratio (RIR) method, chemical methods and the whole
pattern fitting Rietveld method. Of the available methods, the Rietveld
method is probably the most accurate and reliable method. The Rietveld
method is a whole-pattern fitting least squares refinement technique and
has been successfully used for quantification and characterization of
inorganic and organic compounds It has also been used for crystal structure
refinement, to determine size and strain of crystallites.
